The standard approach for the treatment of head and neck cancer (HNC), either induced (HPV+) or not (HPV-) by human papillomavirus (HPV) infection, includes radiotherapy, platinum-based chemotherapy and surgery. However, the combined treatment can cause both acute and chronic severe side effects, underlining the need to explore alternative regimens. Reducing chemotherapy dosage may be a viable option. On this line, previous clinical trials, such as the phase II OPHELIA study, demonstrated safety of the combined administration of platinum-based (cisplatin) chemotherapy along with the PARP inhibitor olaparib before surgery. PARP inhibitors are promising new generation drugs; by acting through the inhibition of the DNA damage repair system, which results in the accrual of unrepaired DNA, making cells more sensitive to DNA damaging drugs, thus enhancing efficacy of chemo- and radio- therapy. PARP inhibitors are indeed successfully used in the treatment of DNA damage repair system-deficient ovarian cancer.
In a recent paper by Citro et al., the authors headed by Susanna Chiocca -PI at the department of experimental oncology of IEO- exploited preclinical models of HPV+ and HPV- HNC to evaluate the killing efficacy of PARPi olaparib in combination with DNA damaging chemotherapy -by exacerbating the chemotherapy-induced unrepaired DNA damage-related stress-, and to unveil the underlying molecular mechanism.
Their work suggests that the two drugs can be safely administered together achieving comparable efficacy to the chemotherapeutic administered alone at high doses, with smaller side effects -confirming the results of previous clinical studies indicating safety of the combined administration of olaparib and chemotherapy- and reveal the underlying molecular mechanism. Although they found greater efficacy in HPV+ cells, the combination therapy was effective also in HPV- cells, proposing the inclusion of PARP inhibitors into platinum-based chemotherapy for an improved, more effective and safer treatment of HNC patients.
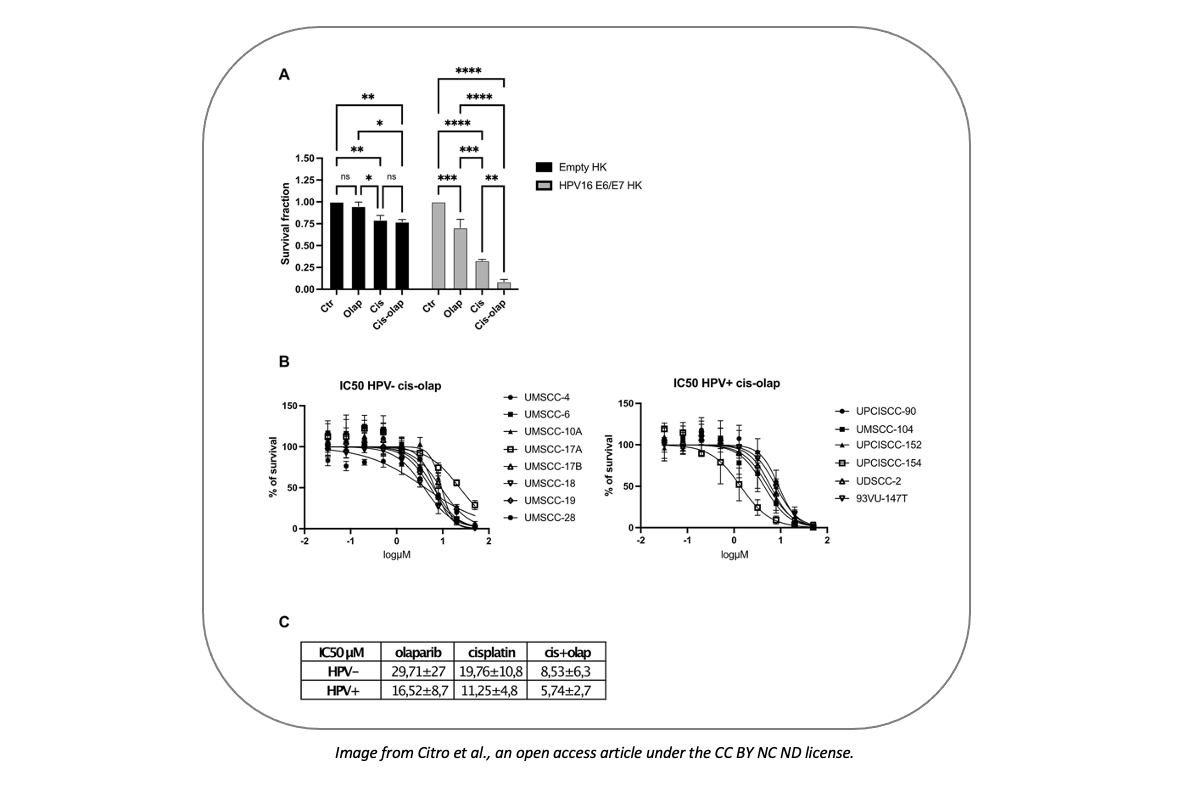
First, the authors focused on HPV+ HNC cells; indeed, these cells show better response to radio- and chemo- therapy, likely due to the impaired DNA damage repair (DDR) mechanism in HPV+ tumors, related to the HPV proteins E6 and E7 interfering with DNA damage repair. Indeed, PARP1 levels (both as RNA and protein) and activity were higher in HPV+ vs HPV- HNC cells, as well as in HPV+ vs healthy surrounding tissue. The increased PARP1 RNA/protein/activity was due to the presence of E6 and E7 proteins: it increased when cells were transduced with E6 and E7 only.
While HPV- HNC cells are usually nearly insensitive to olaparib, HPV infection conferred them sensitivity to PARP inhibition. Cisplatin administration enhanced olaparib efficacy by inducing DNA damage. Both HPV+ and HPV- HNC cells were sensitive to the combined treatment; however, Human Keratinocytes (HK) transduced with high risk E6/E7 HPV exhibited greater sensitivity.
Notably, the two drugs acted synergistically, indicating that the concentration of each of the drugs can be significantly reduced when administered together, maintaining the same efficacy.